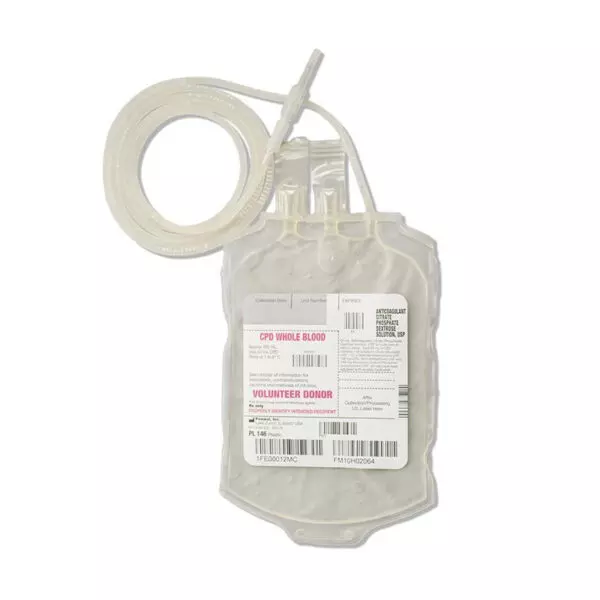
Each blood-pack unit consists of a PL 146 plastic primary container with 63 mL of CPD solution containing 1.66 g Sodium Citrate (dihydrate) USP, 1.61 g Dextrose (monohydrate) USP, 188 mg Citric Acid (anhydrous) USP, 140 mg Monobasic Sodium Phosphate (monohydrate) USP
MEDICAL DEVICE AUTHORIZATION NOTICE: Federal (USA) law restricts this device to sale by or on the order of a practitioner licensed to use or order the use of this device. Purchase of this medical device requires that the user have supervision from a licensed medical practitioner. Devices requiring such supervision may be labeled “Caution” or “RX only”. Ensuring this supervision is the purchaser’s responsibility and the purchaser must acknowledge and accept the terms of this notice prior to sale. Authorization from a medical profession may be requested prior to fulfillment of this item